Excuse my French, but this time we have a breast cancer-focussed study and it’s looking at the role of PICH. There seems to be a stark difference between triple negative breast cancer (TNBC) and other breast cancer types.
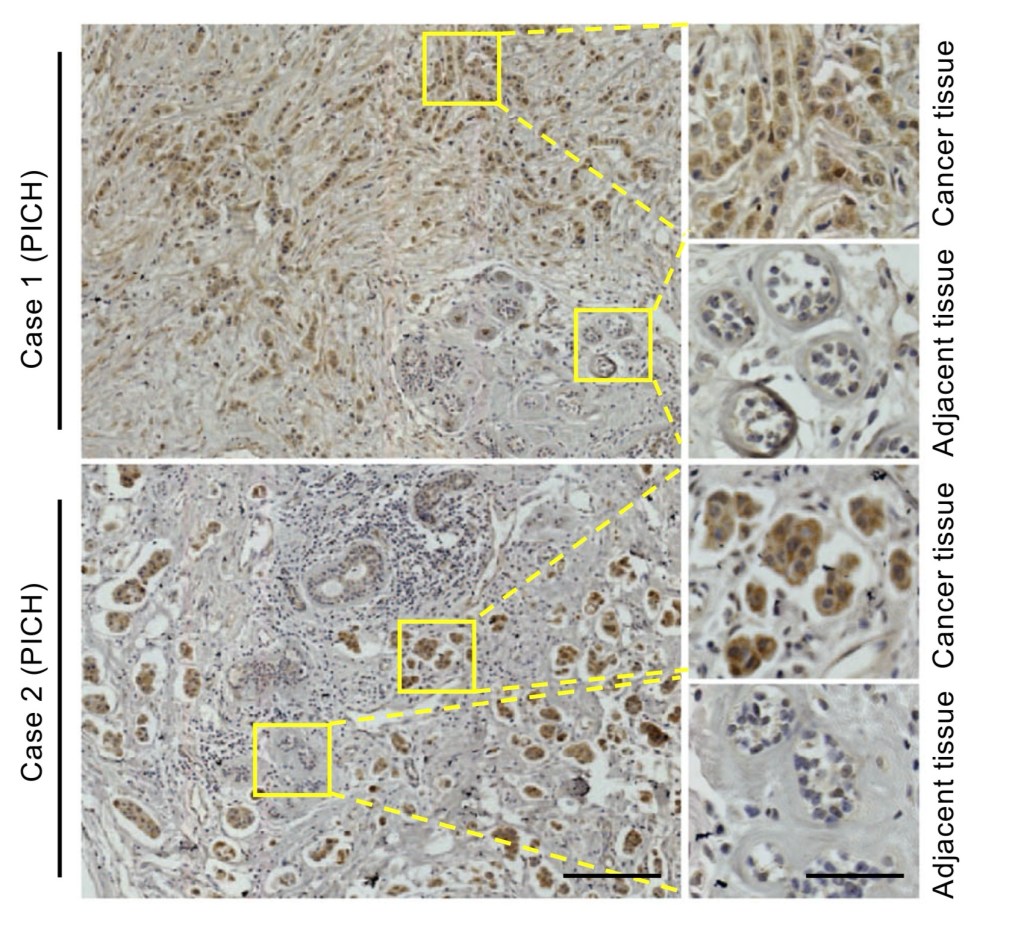
IMAGE: This is figure 1B from Huang et al. (2019), showing PICH expression (brown stain) between cancer tissue and normal adjacent tissue taken from human patients (Huang et al., 2019).
What is TNBC and what are the other types of breast cancer?
When you think of breast cancer, you think of the general disease. It’s a very varied disease, different breast cancers have different characteristics.
There are hormone-positive breast cancers, meaning that they have estrogen (ER) or progesterone (PR) hormone receptors present. This makes them good candidates for chemotherapy that uses these receptors. There are also ERBB2 (HER2) positive breast cancers. HER2 positive cancers can also have hormone receptor expression as well.
Then we get down to triple negative breast cancers (TNBCs). These are very difficult to treat, as they lack PR, ER, and HER2 expression. Current breast cancer chemotherapy is more useful for the luminal types (hormone receptor positive and/or HER2 positive) than for TNBC. When you lack receptors, the drugs have nothing to bind to and activate any of the biochemical pathways.
TNBC tends to be harder to treat because of this, so finding a good therapeutic target is difficult.
What’s PICH?
PICH stands for ‘Plk1-interacting checkpoint helicase’; as the name suggests, PICH is relevant to Plk1. It was initially identified as a Plk1 substrate and binding partner of Plk1. Plk1 is important during mitosis.
It also has ATPase activity (hydrolysing ATP).
PICH Expression in TNBCs vs Luminal BCs
Let’s get down to the study then!
Huang et al (2019) looked at PICH expression in breast cancer tissues and adjacent tissues extracted from 194 human patients. They conducted immunohistochemistry with an anti-PICH antibody in order to observe any expression differences. Immunohistochemistry involves fixing (preserving) the tissues as they are (tissue is dynamic, after all, and not static) and then treating them with stains and antibodies so that differences can be observed under brightfield microscopy. Stains are added generally to highlight parts of the cells (e.g. nuclei) and then antibodies are added to cause a signal to be sent from the the location of the target. Think of it like an emergency flare letting people know of your location.
The researchers found that PICH was highly elevated in the cancer tissue in comparison to the normal adjacent tissue. The adjacent tissue is quite literally tissue taken adjacent to the cancer tissue but is not cancer itself; it’s the person’s normal tissue.
Right, so now we know that the cancer has a difference to the normal tissue, meaning this PICH may be relevant to the cancer growth. One problem: breast cancer is very varied, as we have covered, so are there differences between different types?
To take it a step further and answer this, the researchers also classified each of the breast cancer tissues according to ER, PR, and HER2 expression. They looked at their work again and found that PICH expression was elevated in TNBC tumours compared to other luminal breast cancer types.
PICH is not only elevated in breast cancer compared to normal cells, but it is also elevated further in TNBCs compared to other breast cancers as well.
With regard to survival in patients, patients with higher PICH expression showed a higher risk of metastasis and poorer overall survival than patients with low PICH expression. It seems PICH is elevated in TNBCs and also has a higher risk of metastasis and poorer survival.
To further support their work, Huang et al. looked at 3 database datasets and found that mRNA expression of PICH is generally higher in TNBCs than in non-TNBCs. Another 2 datasets showed increased PICH expression associated with metastasis and poorer survival.
To see if this association is present in vitro as it does in vivo, the study also looked at PICH expression in TNBC and non-TNBC cell lines. They found a higher expression in TNBCs, which confirmed increased PICH in TNBC from the hospital samples as well.
It seemed that PICH expression is unregulated in TNBCs and that higher levels of PICH are associated with poorer patient outcomes and metastasis. Now that this was established, the researchers looked closer at how PICH goes about producing these results.
Impact of PICH Loss on TNBCs
Having established that the cell cultures from before seemed to mirror the patients’ samples, the researchers used cell cultures as a way to assess PICH in TNBC.
They used 5 TNBC cell lines and 4 ER+ cell lines and also generated shRNAs to target PICH. This is a way to knockdown PICH in order to see what the impact of less PICH expression has on the cell lines. It’s a way to see what the relevance of PICH is to TNBC growth.
Huang et al. knocked down PICH and observed cell proliferation 6-8 days later. The TNBC cell lines had a very clear impact on growth and were very sensitive; they did not grow very well when PICH was knocked down. The non-TNBC cell lines did not have a very obvious impact, they continued to proliferate. It seems knocking down PICH impairs TNBC cell growth.
Proliferation
A wild-type (WT) and mutant (mut) form of PICH were applied to a TNBC cell line, MDA-MB-231. The effect of this was observed 7 days after transfecting the different PICH forms. Cells that had PICH knocked down with shRNA can be rescued by WT PICH. When an ATPase-inactive form of PICH was given (it is functional but it’s ATPase function is inactive), no cell proliferation was restored.
An additional observation made during the rescue experiment was that WT PICH remained in the region of the kinetochore during prometaphase. The kinetochore is present at the centromere – where the two chromosome pairs meet. It is a bundle of proteins that aid in the process of mitosis when the chromosomes are being separated. The mut PICH diffused along the chromosome arms rather than concentraing at the kinetochore.
Apoptosis and Mitosis
As we’ve read before, PICH depletion has a big impact on TNBC cell growth in comparison to luminal BCs. Therefore, PICH depletion must have an impact on the cellular processes in TNBC.
The researchers found increased cleaved PARP1 and DNA fragmentation via Western blotting and gel electrophoresis. Cleaved PARP1 is an indicator of apoptosis. The observation was confirmed in apoptosis assays and flow cytometry. PICH seems to be important for chromosome segregation (as we read above) and the increased apoptosis observed when PICH is inhibitied may be due to a disrupted cell cycle progression. If PICH has a role in chromosome segregation during the cell cycle, then it makes sense that low/no PICH will then impact progression of the cycle.
Huang et al. studied further, finding that knocking down PICH induced accumulation of cells with 4n DNA only in TNBC. Normal cells have 2n. Before cell division, the cell replicates DNA to make 4n and then this is separated to make 2 cells with 2n DNA each. The accumulation of single 4n cells indicates that the cell cycle was indeed impacted. This specifically indicates that there was a G2/M arrest (remember the two G2 checkpoints? No? Go read up my last post, I have all the patience and time for you); the cells were able to replicate DNA in S phase but were not able to progress to mitosis and divide. Again, this occurred in TNBCs and not other BCs.
They state that PICH inhibition doesn’t affect the mitotic checkpoint and it only influences chromosome segregation, but we will get to this later…
PICH-silenced TNBCs had no elevated p-H3, which is a G2/M marker in immunofluorescence experiments.
PICH depletion may specifically regulate division in TNBCs.
Chromosomal Instability and Cytokinesis Failure
If PICH impacts chromosome segregation and the cell cycle, it makes a lot of sense that it also impacts the chromosomal stability. Cancers are known to cause chromosomal instability (CIN). This includes heterogenous genomes between cancer cells as well as chromatin bridges, micronuclei, and even binuclei in single cells (Tamura et al., 2020). Could PICH be a reason for this?
Huang et al. looked at PICH knockdowns in chicken and HeLa cells, finding that there was increased chromosomal abnormalities. They found chromatin bridges (bridges between the 2 DNA copies that should be completely separate) as well as micronuclei (tiny nuclei that tend to hang around a cell’s nucleus, kinda like a skin tag).
Upon using immunofluorescence to look at chromosomal changes, the researchrs found that PICH-silenced MDA-MB-231 had higher frequencies of micronuclei, binucleation, and chromatin bridges. Another TNBC cell line, MDA-MB-436, also had higher frequencies of binucleation too. In comparison, luminal BCs with depleted PICH had normal DNA.
Going back to the impact of PICH depletion in apotosis, the study used the MDA-MB-436 cell line using time-lapse microscopy (still using immunofluorescence like a neon highlighter). They wanted to see if mitosis was defective and if apoptosis increased. PICH knockdown was associated with increased cell deaths. To put it into perspective: 3 out of 216 control cells died, whereas 308 out of 387 PICH-silenced cells died. HUGE difference!
Huang et al. hypothesised that cell death was followed by abnormalities in the cell cycle in PICH-silenced cells. PICH is important for chromosomal segregation and chromosomal stability, so a lack of PICH leads to chromosomal instability, which leads to cell cycle abnormalities, which then leads to cell cycle arrest, and eventually cell death. TNBCs depend on PICH for mitosis.
Tumour Growth
Circling back round to the impact of PICH in vivo, the researchers used MDA-MB-231 to produce xenograft tumours in mice. They transplanted cells using either a control shRNA or the experimental shPICH. Mice with the control shRNA (meaning no PICH knockdown) developed tumours within a month, whereas the mice with shPICH (PICH knockdown mice) failed to develop tumours. This validated their work from the patients and cell cultures, showing a reliance of PICH expression in TNBCs. PICH knockdown decreased tumour growth.
They collected these tumour xenografts and fixed them, finding that the PICH-knockdown tumours had decreased proliferative cells and more apoptotic cells. PICH knockdown in luminal BCs did not inhibit tumour growth.
Main Message
Huang et al. looked at PICH, unraveling multiple important roles and impacts of PICH.
In summary:
1. PICH expression is higher in cancer than in normal cells.
2. PICH expression is higher in triple negative breast cancers than in other breast cancers. This is consistent in both patient samples and cell cultures.
3. Higher PICH expression is linked to increased metastasis and a poorer overrall survival in patients with TNBCs.
4. PICH knockdown impacted TNBC growth but not non-TNBC growth.
5. This effect of the knockdown can be rescued with addition of wild-type PICH.
6. Wild-type PICH associates with the kinetochores at the centromeres, mutant PICH moves along the chromosome arms.
7. Increased PARP1 and DNA fragmentation in PICH-depleted cells indicates increased apoptosis.
8. Increased accumulation of 4n DNA cells indicated G2/M arrest in the cell cycle.
9. PICH is important in chromosome segregation in TNBC, so a lack of PICH leads to cell cycle dysregulation and therefore increased cell death.
10. PICH-silenced TNBC cells had higher chromosomal instabilities (binuclei, micronuclei, chromatin bridges).
11. MDA-MB-231 cells with normal PICH expression developed tumours in mice within a month, whereas cells with depleted PICH had failed to develop tumours and had decreased growth.
12. This could have therapeutic advantages, as PICH seems to be important to TNBCs for growth and survival.
My Thoughts
I really loved this paper. The data was presented clear, they didn’t do anything that was not necessary. These researchers laid out a very simple story. It’s somewhat compelling and it is a fascinating read.
1. I have some major thoughts that are making my head spin. The study discusses (in their discussion, of course) that PICH is important with TOP2A function (topoisomerase 2a/topo2a that has a central role in G2 decatenation checkpoint, again, let me guide you there…). PICH cooperates with TOP2A to ensure correct chromosome segregation and PICH can stimulate TOP2A’s catalytic activity. The researchers state that deficiency of PICH can temporarily mimic TOP2A inhibition and that TOP2A expression is higher in TNBC patients as well.
My work on 6 breast cancer cell lines showed that there was a defective G2 decatenation checkpoint. I worked on cell lines including MDA-MB-231 and MDA-MB-436 (as did the study above) and they were very insensitive to the TOP2A inhibitor ICRF193. They continued on into mitosis.
I’m thinking that this may be due to PICH. If PICH is overexpressed in TNBCs and aids with TOP2A function as well as having a role in mitosis, then that may be why my TNBCs were resistant to ICRF193.
The researchers themselves suggested that depleting PICH may increase TNBC sensitivity to TOP2A inhibitors.
2. If TNBCs rely on PICH for chromosomal segregation and stability as well as mitosis regulation, then why is this TNBC-only?
3. Why does TNBC rely on PICH, but not other BCs?
4. They did not look at PICH impacts on chromosomal instability and mitosis in normal cells, as they first did with the adjacent tissue. They need to see what impact on the cell cycle there is in normal cells so that they can see the difference.
5. If PICH is important to the chromosomal segregation (and therefore mitosis), why is there no impact on the cell cycle checkpoint?
6. What about the G2 decatenation checkpoint and DNA damage checkpoints? They should have been more thorough. I worked with TNBCs (MDA-MB-231 and MDA-MB-436) in my MSc, and they had a defective G2 decatenation checkpoint.
7. I really liked and appreciated the multiple links to tissue samples and in vivo experimentation. I think that working solely on cell cultures is helpful only to a certain extent. It’s very difficult to gauge things such as metastasis or survival when you have a 2-D sample.
8. That being said, they could’ve looked at using 3-D organoids to mimic tumours as well. It’s good to cover the bases, and it also may help to get more information about the tumour growth even if it’s not in a patient. I imagine a synthetic sort of mini tumour is helpful to look at than in a patient. You can’t exactly do close-up cell microscopy on a patient.
References
Huang, Y., Li, W., Yan, W., Wu, J., Chen, L., Yao, X., Gu, F., Lv, L., Zhao, J., Zhao, M., Xia, T., Hang, Q., Li, T., Ying, X., Li, T., Xia, Q., Li, A., Zhang, X., Chen, Y., & Zhou, T. (2019). Loss of PICH promotes chromosome instability and cell death in triple-negative breast cancer. Cell Death Dis., [online] 10:428. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6547724/
Tamura, N., Shaikh, N., Muliaditan, D., Soliman, T. N., McGuinness, J. R., Maniati, E., … & McClelland, S. E. (2020). Specific mechanisms of chromosomal instability indicate therapeutic sensitivities in high-grade serous ovarian carcinoma. Cancer research, 80(22), 4946-4959.
Leave a reply to Anthony Cancel reply